By Dr. Arlene Alvarado, Education Coordinator
Question: Many students expressed concern about the ‘dead zone’ on Silver Bow Creek near the waste water treatment plant. They asked, “What do you mean by ‘dead zone’?”
As I wrote in my last column [Montana Steward, 2014, Vol. 4 (1)], for over 100 years Silver Bow Creek was functionally dead, meaning it could not support the biodiversity typical of a Montana mountain stream. The damage to the creek was mostly due to historical mining practices. Silver Bow Creek is doing much better these days, now that mine wastes have been removed, the stream banks have been rebuilt, and the riparian habitat has been revegetated. Silver Bow Creek is certainly showing signs that it is coming back to life as evidenced by trout returning to the creek. However, all is not perfect. Data collected by several researchers, including Beverly Plumb, a graduate of Montana Tech’s Geochemistry Graduate program and her advisor, Dr. Chris Gammons, has shown that Silver Bow Creek (SBC) is suffering from hypereutrophication as it flows between Butte and Rocker. This 4-mile stretch of creek that starts just below the waste water treatment plant has been dubbed the ‘Silver Bow Creek Dead Zone.’ In order to understand what is meant by a ‘dead zone’ in a stream, I will first explain the term ‘hypereutrophication,’ then review some basic chemistry and the biological requirements of aquatic life, most especially, trout. To understand ‘hypereutrophication,’ let’s break this long word down into hyper- and eutrophication. Hyper- is a prefix that means ‘excessive, above normal, high, or over’; so for example, hyperthermia means ‘high or elevated body temperature.’ Eutrophication is a word that originally meant one thing, but has now come to mean something else. Originally, eutrophication meant ‘nutrient-rich’ and was derived from the Greek word, eutrophia which means ‘healthy, adequate nutrition.’ It was used to describe the natural process of aging in lakes or ponds, as the concentrations of plant nutrients built up over very long periods of time. This aging process, however, can be accelerated by human activity that result in the input of nutrients beyond what the aquatic system can handle. Because human-caused eutrophication has become so common, the word eutrophication now has a negative connotation and has come to mean ‘a very harmful increase and acceleration of nutrients’ in water bodies. It is more accurate, however, to call a naturally-aging lake or pond that is nutrient-rich, eutrophic, and a water body that is experiencing excessive nutrients (more than the system can handle) caused by human activity, hypereutrophic.
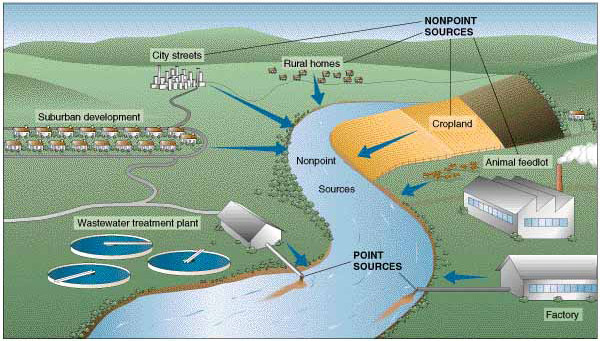
You may now be wondering how hypereutrophication is related to the fact that Silver Bow Creek has a 4-mile dead zone. Put another way, why and how do excessive or high nutrient levels cause a dead zone in the creek? To answer that, let’s review some basic plant biology. In addition to carbon dioxide, water and sunlight, plants need nutrients (food) to develop, grow and reproduce. Three of the most important nutrients plants need are nitrogen, phosphorus and potassium, and these are the three elements you find in most packaged plant fertilizers. Similarly, algae depend on these nutrients for their growth. When excessive amounts of these nutrients enter a water body, such as Silver Bow Creek, algae and plants will respond with rapid growth – much faster than would happen under natural eutrophication. As they say, “Too much of a good thing can be a bad thing.” And this is true of excessive nutrients entering water bodies. Where do the nutrients come from? Excessive nutrients result from many sources – industrial and agricultural discharges into water, fertilized lawns and parks, as well as detergents, animal waste, septic systems, and also from waste water treatment plants that treat human waste. Fertilizer, human waste and animal waste are loaded with high levels of nutrients, such as nitrogen and phosphorous. When this ‘plant food’ enters a water body, it causes rapid growth of algae and aquatic plants, as mentioned earlier. Besides physically interfering with normal aquatic processes, such as blocking sunlight and creating obstacles for aquatic life, this excess in growth of photosynthetic organisms (plants and algae) results in lower-than-normal dissolved oxygen concentrations. Dissolved oxygen is vital for aquatic animals to survive and thrive, especially the gill-breathing insects and fish. Perhaps you are wondering why rapid growth of photosynthetic organisms in water bodies results in decreases in dissolved oxygen concentrations. To answer that, we need to briefly review photosynthesis and respiration in plants and algae. We all know that photosynthesis is how plants and algae make their food. During photosynthesis, carbon dioxide, water and sunlight (photons) are converted into sugar and oxygen. But what happens at night, when there is no sunlight? During the dark hours, plants and algae metabolize (burn) the sugars they created during daylight hours to yield energy for growth, reproduction and other life processes. Like animals, they consume oxygen in order to power their metabolic processes. Therefore, the greater amounts of plants and algae in the water, the more oxygen they consume during nighttime. An overabundance of plants and algae consumes oxygen that would normally be available to fish and insects for their metabolic processes. So, when streams, rivers, lakes and ponds experience hypereutrophication, we see plants and algae consume greater amounts of oxygen, which decreases the availability of oxygen for animals. But it doesn’t stop there. The abundance of plants and algae in the water also means more dead, organic material in the water. Guess what the bacteria that feeds on dead organic matter uses to do their decomposing? You guessed it —
Oxygen!
So, not only are plants and algae sucking up dissolved oxygen from the water, but so are the bacteria that decompose dead organisms. Dissolved oxygen levels decrease so much that the fish and insects literally suffocate. In Silver Bow Creek, a big source of excess nutrients is coming from our waste water treatment plant. Luckily for us and our Silver Bow Creek, Butte’s waste water treatment plant is being upgraded to help reduce or eliminate discharges of excess nutrients. This is great news for our creek. However, another source of excess nutrients is our backyards! When we apply lots of fertilizers to our lawns and gardens, rain storms and melting snow washes lawn fertilizer away and carries it directly into our creek. There are ways to minimize your contribution to excess nutrients in stormwater. For example, the use of liquid fertilizer instead of pellets ensures that the fertilizer gets taken up by the plants in your yard and not just washed away. Additionally, time of year and time of day, and the concentration of the fertilizer are important factors to consider when applying fertilizer and protecting the creek. For more ideas on how you can help to keep our stormwater safe for our creeks, check out the Stormwater insert in this issue.